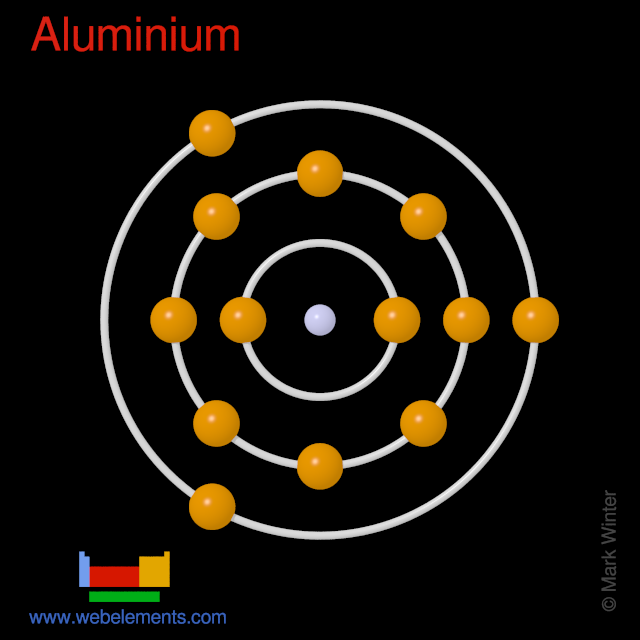
The story of aluminum’s history of use in the U.S. Now stretches over 100 years. The start was a modest one, however. Because of the complexities of refining aluminum from ore, aluminum was considered more rare and precious than gold or silver through most of the 19th century. An atom’s atomic number equal to the atom’s number of protons and electrons. For example: Aluminum’s atomic number is 13, thus each aluminum atom has 13 +(protons) and 13- (electrons). The diagram show s the 13 electrons outside and at different distances from the nucleus. The red circle represent the nucleus and the black circles.
Imagine You Are An Aluminum Atom: Discussions With Mr. Aluminum: I have written a book on aluminium. It is my first book and it will almost certainly be my last.
Pre-publication, the most common question I receive about the book is why I wrote it. I think it began as a reference guide to living in the aluminium age (The Aluminium Age post). An attempt at something akin to frequently answered questions on aluminium and life. Writing the book, putting words on paper, so to speak, changed this. I immediately understood that I could not remove myself from the book. My long affair with aluminium meant that the story had to be personal. I think, I hope, that the reasons for this will become obvious when the book is read.
The book is a warning. When Rachel Carson wrote Silent Spring it was not as a reference guide to pollution of the environment by pesticides. It was a warning. As I wrote my book, I unravelled forty years of research and revealed, at least to myself, that human exposure to aluminium is the, yet unrecognised, unprecedented threat to the future of mankind. Of course, the rallying cry of all scientists remains in that more research is needed. However, the peer reviewed published science is unequivocal in demonstrating the toxicity of aluminium in all living things including humans. The latter most worryingly exemplified by the concentrations of aluminium found in human brain tissue in Alzheimer’s disease, multiple sclerosis and autism to-date (Aluminium in human brain tissue). We have now reached a point where the association of the body burden of aluminium with chronic human disease is unequivocal. We are on a slippery slope and we will continue to slip and slide towards our demise as long as the role, only now, played by biologically reactive aluminium in biochemical evolution remains unchecked. There are solutions and I have written about these in my book. Only the politics of power and high finance prevents ameliorative actions from being taken right now.

Aluminum Atom Mass
I frustrated the publishers of my book by insisting upon a quirky title, ‘Imagine You Are An Aluminium Atom’, they wanted something more digestible (less words) to the book-buying public. I insisted because even though the underlying message of the book is both dark and foreboding, perhaps I should have called it Silent Aluminium, I wanted a title that was peculiar to me (find out why in the book). Books have become integrated into our convenient, throwaway society, the expectation being that, like buses, another will come along in a moment. This book will stand-alone whether it is read or not. It is the story of my life
- Infant vaccines - 23rd April 2021
- Imagine you are an Aluminum Atom - 5th November 2020
- Aluminium in human brain tissue - 8th May 2020
Electron Configurations Worksheet- Examples
- Read my article in Science Education based on my dissertation.

Electron Configuration Notation:
-shows the arrangment of electrons around the nucleus of an atom.
- helps chemist understanding how elements form chemical bonds.
- can be written using the period table or an electron configuration chart.
Aluminum Atomic Number
In order to write the Aluminium electron configuration we first need to know the number of electrons for the Al atom (there are 13 electrons). When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the Aluminium atom.
Aluminum Atomic Mass
In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining electron. Therefore the Aluminium electron configuration will be 1s22s22p63s23p1.
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Aluminum Atomic Number