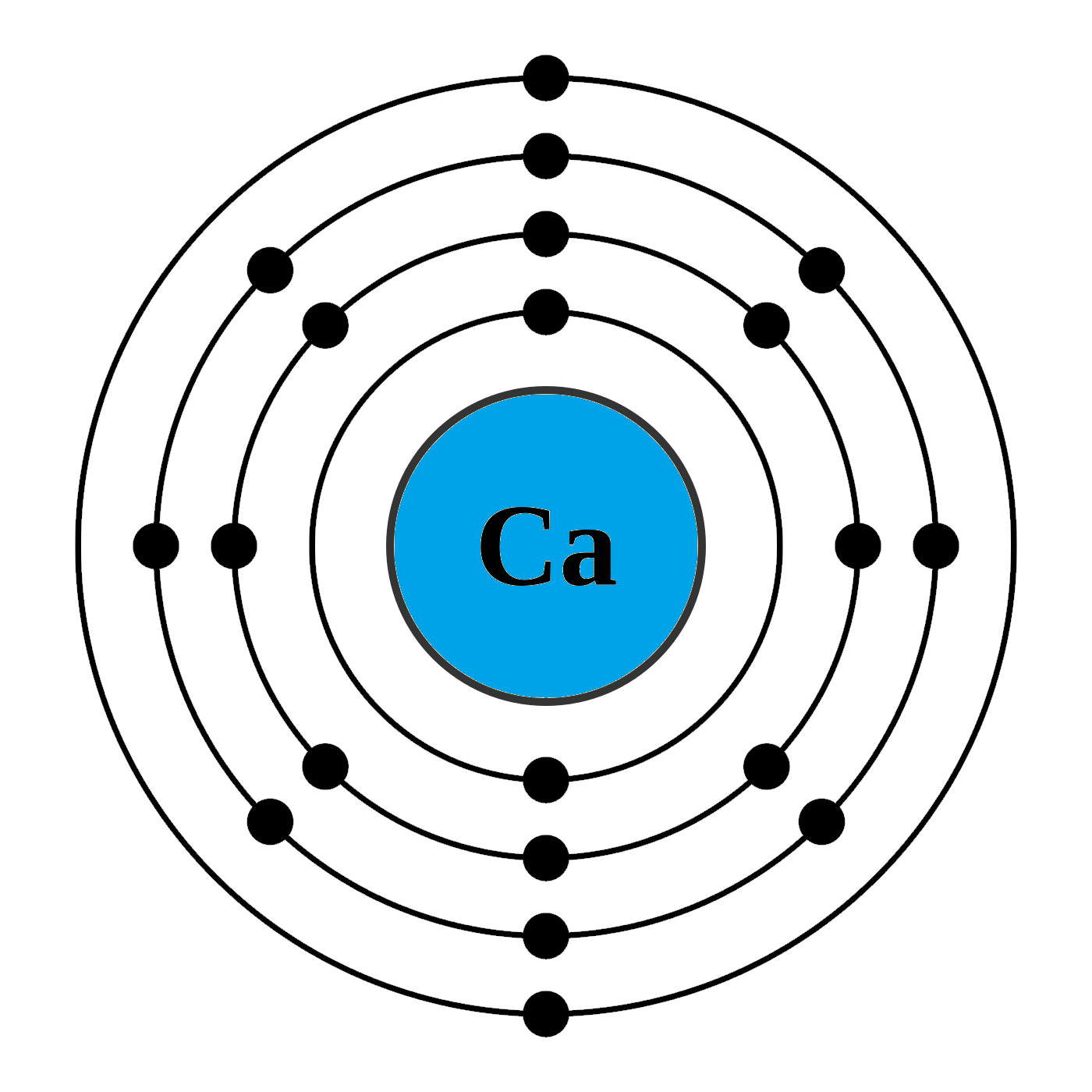
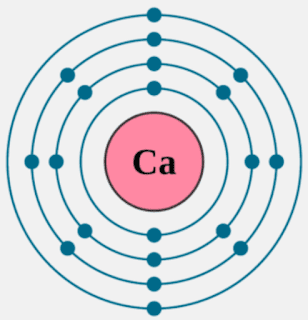
Calcium is an alkaline earth metal and present in the s-block of the periodic table. Calcium lies in 2nd group and 4th period of the periodic table. The electronic configuration of calcium is 4s2. The number of electrons present per shell in a calcium atom is 2, 8, 8, 2. Calcium has two electrons in its outer most shell. Yes, calcium is defined as a metal because of both its physical and chemical traits. They all have an outer shell with two electrons and are very reactive. Those elements in the second column have two electrons ready to make compounds. It shouldn't surprise you that calcium has a valence of 2. You won't find calcium sitting around as a pure. Each subshell is constrained to hold 4ℓ + 2 electrons at most, namely: Each s subshell holds at most 2 electrons Each p subshell holds at most 6 electrons Each d subshell holds at most 10 electrons. Calcium-41 Ca CID 6337034 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety.
Click to see full answer
Likewise, why is a calcium ion smaller than a calcium atom?
Because the calcium ion donates two electrons to achieve the stable state. Thus, the radius of a calcium ion is smaller than a calcium atom.
Likewise, how does a calcium atom become an ion? When it loses electron(s) it becomes positively charged and is called a cation. Calcium atom with electron arrangement K (2),L(8),M(8),N(2) loses two electrons from its outermost shell ( N shell) and forms positive ions called Calcium , Ca2+ ion.
Moreover, what does calcium ion do?
Calcium in biology. Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms cell. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization.
What does a calcium atom look like?
Calcium atoms have 20 electrons and 20 protons. There are 2 valence electrons in the outer shell. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust. Under standard conditions calcium is a shiny, silvery metal.
Click to see full answer
Then, why is a calcium ion smaller than a calcium atom?
Number Of Electrons In Calcium Hydroxide
Because the calcium ion donates two electrons to achieve the stable state. Thus, the radius of a calcium ion is smaller than a calcium atom.
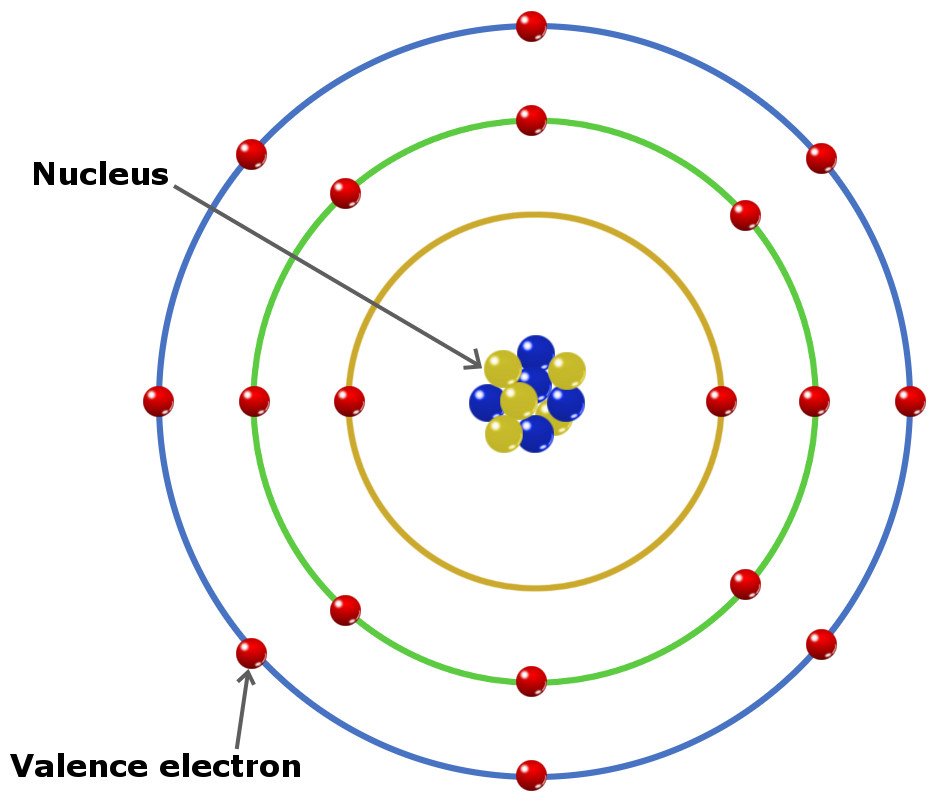
Secondly, how does a calcium atom become an ion? When it loses electron(s) it becomes positively charged and is called a cation. Calcium atom with electron arrangement K (2),L(8),M(8),N(2) loses two electrons from its outermost shell ( N shell) and forms positive ions called Calcium , Ca2+ ion.
Total Number Of Electrons In Calcium
Then, what does calcium ion do?

Calcium in biology. Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms cell. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization.
What does a calcium atom look like?
Calcium atoms have 20 electrons and 20 protons. There are 2 valence electrons in the outer shell. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust. Under standard conditions calcium is a shiny, silvery metal.
